The security police, who are responsible for maintaining security during the demonstrations that are taking place in our country, may have been targeted by jets of corrosive products such as strong acids. This is an opportunity for us to review chemical burns: how do the products work, what are the emergency treatments?
A glimpse of history
On the one hand, there are accidental exposures to corrosive household products (strong acids for descaling, strong bases for unclogging pipes or cleaning ovens…), or to professional products in the workplace such as hydrofluoric acid (petroleum industry, glassmaking, metallurgy, uranium), acids for batteries, bleach… Children are generally burned in the family environment during their so-called “exploration” phase. Direct contact between child and product is sometimes obvious (skin or eye contact) but it can sometimes be hidden when the product has been ingested. Adults are victims both at home and at work.
On the other hand, burns can occur as a result of acid jet attacks, for example. These violent demonstrations have been known since the mid-19th century but are on the increase. Previously known as “honor killings”, they may be committed by jealous men or pregnant women outside marriage (less frequent).
Currently, the number of other types of attacks is increasing: in 2016 in Great Britain, 700 cases were reported, including 431 in London. They are the result of gang competition and delinquency: men are more frequently affected than women, unlike in previous cases. In June 2017, a 47-year-old British nurse died after being sprayed with acid. She died of sepsis.
In Europe, the number of burned or disfigured victims has increased dramatically in the last ten years. More than 2,600 acid attacks were carried out between 2015 and 2018, an average of two every day.
In France, 4 American women were assaulted at St Charles de Marseille station in 2017 and 3 people in the Paris metro in February 2019. This weapon, accessible to all and available over the counter, could have been used against the police during the weekly violent demonstrations currently taking place in France.
Which corrosive products are most often implicated?
- acids: sulphuric acid, nitric acid, hydrofluoric acid, hydrochloric acid, acetic acid, formic acid, phosphoric acid, phenols and chloroacetic acid ;
- bases: sodium hydroxide, calcium hydroxide, sodium and calcium hypochlorite, ammonia, phosphates, silicate, sodium carbonate, lithium hydride ;
- oxidants: bleaching agents such as chlorites used in the home, peroxides,
- others: white phosphorus, metals, hair dyes,
- vesicants such as mustard gas.
Physiopathology
Type of burn
Chemical burns are rare (1-2% of burns) and most often ocular (50% of chemical burns), but unlike a thermal burn, chemical burns continue to evolve until they are chemically neutralized.
The danger is that only a simple, non-specific treatment of the causative agent will be carried out, leading to an increased risk of systemic toxicity (through the skin or by inhalation).
The severity of a chemical burn is directly related to:
- the nature of the product (penetrating power and mode of action);
- the amount of product;
- the concentration of the product used;
- the duration of contact between the skin and the chemical;
- the nature of the exposed tissue; the eye is more fragile than the skin; toxicity by absorption by a mucosa is very important…
Modes of action
Six mechanisms predominate:
- oxidation-reduction, the chemical causes cell destruction;
- desiccation, dehydration processes;
- the exothermic reaction causes thermal burning;
- saponification of fats, skin lysis due to alteration of fatty elements;
- coagulation of proteins, liquefaction of proteins.
Acid burns
The main mechanisms of action of acid burns are dehydration, protein coagulation, and exothermic reaction. Lesions are most often well defined and not very deep with dry necrosis resulting in less exudation, reduced risk of infection and slow detersive action. In 76% of cases, chemical burns are caused by sulphuric or nitric acid.
- sulphuric acid H2SO4. Sulfuric acid is the most dehydrating. It gives black or brownish, dry, hard and painless necroses. The exothermic reaction is strong. There is a risk of systemic passage with glottis edema and shock,
- nitric acid HNO3. It is a liquid at ordinary temperature but it produces toxic nitrogen oxide fumes that can cause corneal and lung burns, sometimes delayed by 5 to 48 hours. Necrosis is yellow in appearance,
- hydrochloric acid HCl. It causes white necrosis. It induces a significant gas release that can cause tracheal and bronchial epithelial necrosis.
- The special case of hydrofluoric acid (HF). It is liquid at low temperature and gaseous at ordinary temperature. After a period of latency due to a transient anesthetic effect of a few hours, very deep and painful burns develop. Its mode of action is specific. Hydrofluoric acid has a double action: corrosive, bound to the H+ ion which attacks tissues on the surface, then toxic, bound to the fluorine ion which penetrates cells and binds to calcium and magnesium (chelation) causing cell death. This results in a significant release of potassium. Lesions are liquefaction necroses close to those of the bases. There is then a lethal risk of hypocalcemia, hypomagnesemia, and hyperkalemia.
Alkaline burns
They act by destroying proteins and collagen with cellular dehydration and caloric release. Lesions are liquefaction necroses with irreversible changes in the protein matrix and local or general vascular damage. Liquid losses are very high. Eye damage is very serious. The main agents responsible are NaOH and ammonia NH4OH, which emits very irritating gases. Alkaline agents are generally more toxic than acid agents due to the irreversible destruction of proteins.
Emergency measures
Skin
Remove clothing and all contaminated objects such as rings and other jewelry; it is necessary to remove the corrosive product from the skin as soon as possible.
As we have already read in the blog on dry decontamination [1], the absorption of the product by dry absorbent support considerably reduces the harmful effects of the product, if only to avoid spreading the product.
On the other hand, irrigation with water is essential.
We often talk about the three 15 rule.
Reading the various publications, we notice that the number of 15 is much higher than 3!
- irrigate for at least 15 minutes to cool the wound, ease pain, limit swelling and deepening of the burn
- with water at 15°C (to avoid hypothermia);
- over an area not exceeding 15% of the entire body (to avoid hypothermia);
- by rubbing water 15 cm from the wound;
- required within the first 15 minutes after contact (actually as soon as possible) ;
- and call 15 !
Do not use neutralizing products such as a basic product to neutralize acid or vice versa.
When hydrogen fluoride is involved, a specific treatment is possible combining early and prolonged washing with water and the administration of calcium gluconate. Gluconate can be administered as a 2.5% gel or as a 10% subcutaneous injection (0.5 mL per cm2 of the burned area) in burned areas. In the case of necrosis, surgical excision will be performed.
Eye
When the eye is reached its thorough rinsing with water, including under the eyelids is essential.
Note the highly discussed place of Diphoterine ® as a decontaminant of the skin and eyes. This product is amphoteric and hypertonic but we do not know its composition. Its hypertonicity would prevent the chemical from penetrating into the tissues. It would have a certain and fast analgesic virtue. Its chemical binding energy with the agents would be higher than that of the tissue receptors. It would act on chemicals through a non-exothermic reaction. However, scientific studies show a lack of objective data demonstrating the superiority of Diphoterine ® over water washing. This product does not have the status of a drug but only a medical device and its clinical efficacy has not been proven.
To be exhaustive, when faced with a chemical burn, whatever the dilution of the causal agent, the duration and/or the contact surface, the question of the risk of systemic toxicity must always be considered.
The following table from reference 5 provides a good overview of the lesions and toxic effects of common products
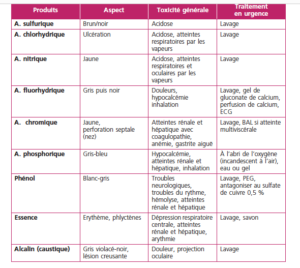
Ingestion
When ingested, no emetic or neutralizing agents that could aggravate the situation.
In the case of button cell batteries (especially by children), they are likely to release alkaline products causing local burns, usually esophageal. This requires endoscopic management and radiographic follow-up. Early removal is strongly recommended. If the battery has passed through the pylorus, wait patiently and be sure to watch for it to appear in the stool.
The interest of Decpol ABS® wipes [2]
Decpol ABS® wipes are essential to limit the spread of chemicals on clothing and skin. These systems prevent spreading and stop the penetration of chemicals into the skin. The simplicity of use and the small size, in particular, Decpol ABS® wipes, make it possible to be efficient by intervening quickly: this is a key factor in limiting the severity of chemical damage. Note that the wipe is part of the “Emergency Protection Kit”.
References
1- Angéline Plebanskyj. Attaques à l’acide. Éviter le pire grâce à un geste simple. (https://www.alternativesante.fr/fre/22/article/journaux/achat_journaux/56/4547 [3])
2- Tess B. VanHoy, Michael H. LeWitt, Bhupendra C. Patel. Chemical Burns. NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 1 mars 2019.
3- A. Breden, J. Laguerre. Brûlures chimiques cutanées ; mécanismes et prise en charge. Le point du vue du brûlologue.
www.toxicologie-clinique.org/stc2009/conferences/breden.htm [4]
4- F. Testud. Brûlures chimiques cutanées et oculaires : mécanismes et prise en charge
Le point de vue du toxicologue. http://toxicologie-clinique.org/stc2009/conferences/testud.htm [5]
5- E. Bourgeois, MR Losser. Brûlures graves. Chapitre 72. SFMU, SAMU
Autor: Prof. François Renaud