When in France the challenge against vaccines is still valid, we are going to focus on a vaccine which obligation was repealed in France in 1984: the smallpox vaccine. In fact, if smallpox is eradicated from the surface of the globe (the last case was reported in Merqua, Somalia, in October 1977), justifying the end of the vaccination obligation, this disease represents a potential danger since it is still regarded as a major biological weapon in the NRBCe, classified as category A. Smallpox vaccine is therefore still relevant. But, let’s reassure us, it’s not one of the 11 mandatory vaccines that make a lot of talk about them right now!
History
The large resemblance of viruses such as monkeypox (monkey), cowpox (bovine), or camelpox (camel) shows that the disease appeared in the Neolithic from the domestication of animals at the time of sedentarization (4th millennium BC). The cowpox virus could be very close to the common ancestor of all these viruses. It is found in Antiquity in the Middle East (Egyptian mummies are marked by the disease). It is present in India, China and the Roman Empire, where it has certainly caused several epidemics. But, for the first time it was well-defined in France and Italy in 570, and it was Marius, Bishop of Avenches (or Marius d’Avenches) who first used the term “Variole” (small pox) to describe the epidemic which was raging that year.
 Ramses V mummy with signs of smallpox pustules.
Ramses V mummy with signs of smallpox pustules.
Very contagious, the smallpox is due to an orthopox virus. During centuries it raged on all the continents, decimating the populations. It is doubtless one of the most murderous diseases that knew the humanity.
Disease
Symptoms
The virus remains silent during the incubation period of 12 to 14 days. The onset of the disease is sudden: flu-like symptoms, fever, headache, prostration … Then the temperature drops, and the characteristic eruption appears on the face, hands, forearms and then later on torso. The vesicles are filled with a clear liquid, which is transformed into pustules containing pus, which are then transformed into crusts which dry and separate. Smallpox was fatal in 30% of cases.
Transmission
It is an exclusively human disease. Transmission is via the respiratory tract (drops of saliva, aerosols) and skin contacts with the pustules. Dried crusts found in clothing are also infectious.
Death
It comes because of the accumulation of toxic products in the blood resulting from the multiplication of the virus. This causes acute lung edema, septic shock or cardiovascular collapse. Before the antibiotics, bacterial superinfection of the skin or lungs increased the mortality of the virus. In the vast majority of cases there are still important sequelae as indelible marks on the face.
Disease prevention
Variolation
The people who contracted the disease but who did not die are protected several years, even decades. Thus the variolation consisted in inoculating a healthy subject with the contents of a vesicle of a weakly reached sick person or himself variolated to protect him against the smallpox. We thought that this form of disease was little virulent and that it could protect the subjects in an effective way. The result remained nevertheless random and risked, the mortality rate having been calculated to 2 %.
This practice was certainly born in China and it propagated along the silk route. The technique is imported in West by at the beginning of the XVIIIth century by Lady Mary Wortley Montagu, wife of the ambassador of England in Turkey which has variolated her own children. In some rare exceptions, this risked controversial method, accused of provoking epidemics (the inoculated people being contagious), was never practiced in the French population.
Principle of vaccination
Edward Jenner (1749 – 1823) was an English scientist and physician.
Starting from the observation that the farmers who milk the cows generally don’t contract smallpox, he theorized that the pus present in the vesicles of women who contracted vaccinia (a smallpox-like but much less virulent disease) protected them. Indeed, vaccinia virus (cowpox) is very similar to smallpox virus and gives a benign transmissible disease which can be the cause of a protective cross-reaction against smallpox.
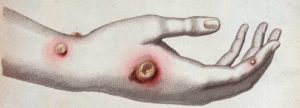 Cowpox on the hand of a farmer
Cowpox on the hand of a farmer
In 1796, Jenner tested his theory by inoculating James Phipps, an eight-year-old boy, with the contents of vaccinia vesicles from the hand of Sarah Nelmes, a farmer who contracted vaccine transmitted by a cow. Later, he “variolizes” (with smallpox virus) the young boy who did not react at all: he was therefore well protected against the smallpox virus. Protection was achieved by injection of vaccinia or “vaccination”. This type of protection will then be named “Jennerian vaccination” by Pasteur (later, simplified to become “vaccination”). Jenner keep on his work and vaccination was accepted. In 1840 the British government banned the variolation and encouraged free vaccination. It then spread throughout Europe.
It should be noted that the transmission of the vaccine was carried out for a long time directly from arm to arm, but it was observed that this, not only caused a reduction in the efficacy of the vaccine but also allowed the transmission of diseases such as syphilis. After that, the virus was then taken directly from a cowpox sick bovid.
In fact, the vaccine virus which was subsequently used, in particular for the eradication of the disease, is not cowpox virus (CPXV) such as that used by Jenner but vaccinia virus (VACV), which is immunologically very close.
Vaccine and the eradication of smallpox
By the end of the 1960s, smallpox was still endemic in Africa and Asia, so it was in these regions that WHO’s efforts to combat this terrible disease were concentrated. The effectiveness of mass vaccination of populations did not produce the desired results, and it was by another so-called “containment-monitoring” method that smallpox was completely eradicated. This long and meticulous process consisted of isolating the patients and vaccinating all their contacts in order to break the transmission chain. In 1980, the Director General of WHO proclaimed the global eradication of smallpox.
 Vaccination against smallpox in 1905
Vaccination against smallpox in 1905
The first compulsory vaccination in France
The Act of 23rd December 1874 (Roussel Law) forces nurses to be vaccinated against smallpox. In 1902, France introduced the first compulsory vaccine, in this case the smallpox vaccine. The suppression of vaccination against smallpox in babies occurred in 1979. This decision was made after several accidents caused by smallpox encephalitis. The risk of vaccination was then considered to be greater than the risk of disease. Removal of re-vaccination occurred in 1984.
Smallpox vaccines
First generation vaccines
These are the traditional historical vaccines made up of live replicative viruses. These vaccines contain vaccinia viruses maintained on animals or eggs. They can be the cause of post-vaccination complications and no longer meet the standards of good manufacturing practice, so they no longer have marketing authorizations. But important reserves have been created and they can be used in case of major need.
Second generation vaccines
These are the same viral strains that are used but they are produced on cell cultures.
Two types of vaccine have been developed: a monoclonal vaccine after selection of an attenuated viral clone and a polyclonal vaccine respecting the antigenic diversity of the initial virus.
The only approved second-generation vaccine is the ACAM2000 monoclonal vaccine currently manufactured by Sanofi Pasteur Biologics and consists of a viral clone grown on Véro cells. Currently licensed in the United States in Australia and Singapore and has been administered to more than 1 million people. It has few post-vaccination complications.
Third generation vaccines
These vaccines are also live vaccinia viruses produced in cell culture. Having lost several genes they are attenuated and are no longer able to replicate in human cell lines. This is the case of the Imvanex /Imvamune vaccine manufactured by Bavarian Nordic. This vaccine is registered in Europe and Canada. When administered to more than 7 600 people, it didn’t give post-vaccination complications.
These two types of vaccine have shown a protective power in animals.
First-generation vaccines induce 80% protection, and 95% in patients who have been revaccinated several times. After exposure, a vaccination is able to protect when administered within 3 to 4 days of exposure. In France, suspension of primary immunization occurred in 1978 and recalls in 1984. In 2004, it was estimated that more than half of the population had never been vaccinated (much more in 2017) and that the other half had an old protection. The immune vulnerability of the population to reintroduction of the disease is therefore a cause for concern.
Post-vaccination risks
Smallpox vaccine is highly reactogenic, especially in primary vaccines. It can therefore have a number of side effects that can be severe.
Some accidents are frequent but reversible, such as accidental inoculation and generalized vaccinia.
Accidental inoculation is an contamination away from the vaccine lesion generally located at the level of the eye or the perineum in the vaccinated subject or even on the skin of the contacts of the entourage of the vaccinated because the vaccine pustule is relatively contagious.
The generalized vaccinia is the appearance a few days after the inoculation of an eruption covering sometimes the whole body: the prognosis is good.
Much more dreadful with fatal risks are vaccine eczema, progressive vaccinia and post-vaccine encephalitis.
Vaccine eczema has a frequency of 1.5 to 4.5 cases per 100 000 primary vaccinations, of which 6% are fatal. This complication occurs in patients with eczema or with a history of eczema: eczema and intense inflammation can be particularly severe if the injured surface is important.
Progressive lethal vaccine (vaccinia necrosum) occurs in immunocompromised individuals: the vaccine lesion does not recover, secondary lesions occur throughout the body and death can occur within 5 months of vaccination.
Post-vaccination encephalitis in 1 case per 100 000 primary vaccinated predominantly infants, it is fatal in 30% of the cases and sometimes leaves heavy consequences in the survivors. It is characterized by seizures in children younger than 2 years of age or by fever, vomiting, headache and unconsciousness, confusion and even coma in children over 2 years of age.
There may also be myocarditis and pericarditis.
Management of vaccine accidents
The treatment of post-vaccination accidents can be carried out by the administration of anti-vaccinia immunoglobulins. These immunoglobulins consist of hyperimmune plasma collected from recently vaccinated volunteers. The stock consists of individual pockets kept in the cold.
Emergent BioSolution Inc. is marketing the “Vaccinia Immune Globulin Intravenous (human) = VIGIV.” This product, also known as CNJ-016, was approved by the FDA in 2005 and by the Canadian Department of Health in 2007. It consists of sterile solution of immunoglobulins (> 96%) containing vaccinia virus antibody antibodies stabilized by maltose and polysorbate 80. It contains no preservatives and can be kept for up to 10 years at -15 ° C. Ig originates of selected donor plasmas just undergoing a booster vaccination to boost their antibody titer.
VIGIV is indicated for the treatment of vaccine complications of smallpox: vaccinia eczema, progressive vaccinia, generalized vaccinia, accidental inoculations (except keratitis or even contraindicated) and specific skin problems. It is not effective against encephalitis. The product is injected intravenously.
Application to bioterrorism
The threat of a smallpox epidemic is low but it is seriously taken in consideration by French state agencies. Vaccine stocks are sufficient for the French population. The procedures are in place in the biotox plan which defines 5 levels of alert.
Vaccines belong to the first generation and post-vaccine complications are known to be of the order of 14 and 52 cases of side effects /million with the death of 1 to 2 people/million (OMS). So, in the event of an attack, it will be necessary to ask the question of the benefit/risk ratio. Other publications show that the postvaccinations accidents can be more important : 1/800 for primo-vaccinations and 1/10 000 for re-vaccinations (Lane et al.).
Among the second-generation vaccines, ACAM2000 is the only one approved by the FDA. It is to be bought from Sanofi by Emergent BioSolutions Inc. Administered to 1 million people, no severe sequelae have been reported except for myopericarditis. It therefore gives fewer side effects and is especially less much less neurovirulent than the first generation vaccines.
The Imvanex/Imvamune vaccine manufactured by Bavarian Nordic is of the 3rd generation. It is unable to reproduce in human cell lines. Administered to more than 7,600 people, including HIV-positive people and patients with atopic dermatitis, it has been well tolerated. The United States ordered 20 million doses in 2009 and took an option of 60 million doses.
However, no data were provided on children and pregnant women. Their protective power has been demonstrated only in animals.
In France, the vaccination strategy is decided by the SGDSN (Seretariat général de la défense et de la sécurité nationale). The HCSP (High Council for Public Health) recommended in 2012 to revise the smallpox plan by constituting for example a stock of 3rd generation vaccines for first responders, ie 250 000 doses for vaccination of 125 000 people. The file is classified as “confidential”.
Conclusion
In the face of a possible smallpox threat, vaccination is the only operational preventive measure. It should be restrictive and modulated according to the supposed epidemic risk compared to the risk of post-vaccination complications. The warning system must be effective as well as the training of medical personnel, the vast majority of whom have never been confronted with this disease.
Bibliographie
M. Rey, N. Guérin. Variole et menace bioterroriste. Antibiotiques, 2004, 6, 214-216
J. M Lane, F. L. Ruben, J. M. Neff, J. D. Millar. Complications of Smallpox Vaccination, 1968: Results of Ten Statewide Surveys. J. Infect. Dis. 1970, 122, 303-309.
OMS. Innocuité des vaccins antivarioliques [1] (lu le 26/09/2017)
OMS. Initiative mondiale sur la sécurité des vaccins. Sécurité des vaccins antivarioliques : questions et réponses [2](lu le 26/09/2007)
Emergent biosolutions. Emergent BioSolutions to acquire ACAM2000 business from Sanofi [3] (lu le 26/09/2017)
Julie Dimier. Développement d’un vecteur virus de la vaccine, réplicatif et attenué, pour la vaccination antivariolique et pour la vaccination contre la fièvre hémorragique à virus Ebola. Sciences agricoles. Université de Grenoble, 2012. Francais.
La variole a été éradiquée grâce au vaccin antivariolique [4] (lu le 26/09/2017)
J.D. Shearer, L. Siemann, M. Gerkovich, R. V. House. Biological activity of an intravenous preparation of human vaccinia immune globulin in mouse models of vaccinia virus infection. Anti. Agent Chemother. 2005, 49, 2634-2641.
J. Freney, F. Renaud. La guerre des microbes, de l’antiquité au 11 septembre 2001. Eska ed. 2009, 167 p.
Et sur le site Ouvry :
Et l’on reparle de la variole [5]
On a retrouvé des flacons de variole [7]
Encore les souches du virus de la variole [8]
__________
Autor : Prof. François Renaud