Current images show that tear gases are massively used to maintain order during demonstrations. This leads us to ask ourselves some questions to better understand these products: what is their composition, how did they appear, what are their mode of action and toxicity, how can they be protected or even eliminated, what is the legislation regarding their use? A quick overview!
What are we talking about?
A tear gas is an agent that causes irritation of the eyes (tear flow) and/or respiratory system causing temporary disability. It is a chemical substance chosen for its low toxicity and is therefore considered a non-lethal weapon.
Historical background
The first known tear gas is ethyl bromoacetate, known since 1850 for its irritating properties and used by the Paris Police Headquarters from 1913 to neutralize recalcitrant individuals. The French army used this product in the form of suffocating grenades or Bertrand grenades in August 1914 against the German army but without ever causing death (minor tear gas). The German army responded with the same irritant gas, called Niespulver (sneezing powder) on 27 October 1914 by firing 3,000 shells in the Neuve-Chapelle sector. It was a complete failure. But, if we consider that the use of this gas during a conflict constitutes an act of chemical warfare, we must admit that it was the French who fired first… The debate remains open!
Chemical composition
There are several tear gases: chlorobenzylidene malonitrile (also known as “CS” after the names of its inventors, 2 scientists from Middlebury College (Vermont, United States), Ben Corson and Roger Stoughton who developed it in 1928, chloroacetophenone (“CN”), dibenzoxazepine (“CR”) and OC pepper (Capsicum oleoresin). CS is the most widely used product.
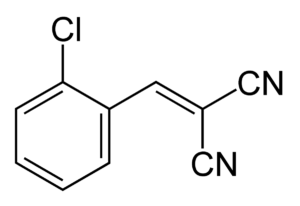
Clinical effects
CS is a white crystalline powder with a peppery odour, insoluble in water. Because of its use for crowd dispersal, toxicity to humans is almost exclusively acute. The irritant effect is clear and manifests itself on the eye (tearing, conjunctivitis, sometimes photophobia), on the skin, on the respiratory tract (rhinorrhea, cough, dyspnea and chest pain). Digestive disorders (nausea, vomiting and diarrhea) and headaches may also occur. The effects cease within half an hour after the product is removed. Sometimes an allergic effect can occur.
In high doses, burns up to second degree, corneal damage, necrosis of respiratory and digestive tissues and pulmonary oedema may occur. This is the case for use in a closed environment. Cases of secondary transmissions on medical personnel have been described.
First aid services
The product is in powder form and as with any dust-generating substance, the risk of dust explosion in contact with an ignition source must be taken into account. In addition, the heated product breaks down into toxic products such as hydrogen chloride, nitrogen oxides and carbon monoxide. First aiders must therefore be equipped with autonomous individual respiratory protective equipment.
Utilization
In addition to CS there are modified versions: CS1: composed of 95% CS, added to 5% silicate gel (low density materials) to prevent CS particles from coagulating, CS2 : composed of 94% CS, 5% colloidal silicate and 1% bis(trimethylsilyl)amine (solvent), it is designed to float on water and resist degradation, CSX : liquid version of CS, it consists of 1% CS1 and 99% trioctylphosphite (stabilizing agent). These 3 forms have different physicochemical properties.
The product is used as grenades or sprays by police forces. A grenade can be thrown by hand between 15 and 20 meters and at 200 meters with a Cougar. CS produces an incapacitating physical impairment. The use for law enforcement purposes is subject to strict rules such as obtaining the agreement of the civil authority (prefect) by the company commandant.
Self-defence
Personal tear gas can be sold for self-defence purposes.
Most tear gas canisters are classified in the 6th category, of the general classification of weapons and ammunition except in the case of CS-based presentations, concentrated at less than 2%, whose filling volume is less than 100 mL and whose valve flow rate is less than 60 g/second. In any case, the sale is prohibited to underage people.
There are 2 types of presentations: aerosol (spray) to neutralize several individuals at the same time due to the extreme volatility of the product. There are also gel presentations that liquefy instantly upon contact with the skin and mucous membranes, allowing it to attach only to the aggressor without spreading into the room.
Are tear gas chemical weapons?
The Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons on Their Destruction, opened for signature in Paris in 1993, which entered in force in April 1997 and was ratified by 193 countries, stipulates that each State Party undertakes not to use riot control agents as a means of warfare (any chemical product that is not listed in a schedule and that can rapidly cause sensory irritation or physical disability in human beings that disappears shortly after exposure has ceased). So tear gas is considered chemical weapons if it is used as a method of warfare. They are therefore prohibited during conflicts between countries. On the other hand, they are allowed within a country, which seems quite paradoxical.
Why?
The fact that tear gas was used to take soldiers out of trenches or bunkers (especially during the First World War) and then attacked them with firearms or other gases is unacceptable. In addition, on a battlefield, it is impossible for a soldier to distinguish an irritant gas from a deadly gas. And finally, the physical charges of police officers, to disperse a crowd, water cannons (sometimes with tear gas or olfactory additives), rubber bullet guns or even blast grenades, present risks of physical injury. If tear gas is far from perfect, it continues to be used because there is nothing better! (Pr. D. Koplow).
The prevention
Wearers of contact lenses must remove them or risk seeing the product positioned under the lenses at the risk of damaging the eye.
To minimize the effect of tear gas, the best protection is the gas mask with the correct cartridge.
Gas masks are considered as war material. According to the Internal Security Code, “it is prohibited for individuals to acquire or hold war material falling within category A2”. There are derogations for certain professions. For example, people who work in contact with toxic products can obtain them. In addition, a mask makes it difficult to identify a person. According to the Circular of 2 March 2011 relating to the implementation of Act No. 2010-1192 of 11 October 2010 prohibiting the concealment of the face in public spaces, any clothing or accessories that “make it impossible to identify persons” is prohibited.
That is why we see demonstrators wearing ski or diving goggles to protect their eyes and surgeon’s masks for their lower faces.
The company Ouvry provides professionals with masks and effective cartridges
https://www.ouvry.com/produit/masque-nrbc-oc50/ [1]
https://www.ouvry.com/produit/cartouche-polyvalente-milcf50/ [2]
https://www.ouvry.com/produit/abek2-p3-nbc-spectre-large-militaire-civil/ [3]
First aid measures
In case of exposure, do not rub your eyes, which increases tears. Rinse eyes thoroughly with physiological water. If undressing is necessary, avoid the passage of clothing through the head and be careful of possible cross-contamination.
The decontamination
The decontamination of a place in which tear gas has been spilled may sometimes be essential (a closed room for example for quick and safe reuse). The product DesDec from Ouvry https://www.ouvry.com/produit/desdec/ has experimentally shown its effectiveness in this kind of situation
References
Tear gas, Wikipedia, accessed January 5, 2019.
INRS, MSDS database, website www.inrs.fr/fichetox, accessed 02/2016
https://www.ouestfrance.fr/leditiondusoir/data/39949/reader/reader.html#!preferred/1/package/39949/pub/58046/page/8
Photo, Jacques Pezet, “Libération”
Autor: Prof. François Renaud