As the Covid-19 pandemic is still spreading across our territory, we will be looking at the various treatments currently being tested and potentially capable of stopping the progression of the disease in humans.
The disease
The surprising thing about Covid-19 is the extreme variability of the disease. Classic clinical signs appear after an incubation period of 1 to 14 days (in most cases around 5 days). There is fever around 38°C, a dry cough sometimes irrepressible and great fatigue accompanied by muscular pains. Shortness of breath and breathing difficulties may appear around the 7th day of the illness. In some cases there is a loss of taste and smell (anosmia) which is quite characteristic of the disease. In some patients, around the 10th day, there may be an abrupt deterioration of respiratory functions leading to severe acute respiratory syndrome while the viral load seems to decrease: this is due to an inappropriate response of the immune system in the lungs which over-reacts to the presence of the virus.
A significant number of individuals do not express any symptoms while spreading viruses around them: they are healthy carriers. Clinical signs may be missing, while less common ones such as diarrhea may be seen.
The goal of treatment is to lower the viral load at the beginning of the disease before severe respiratory symptoms appear.
The virus
Structure
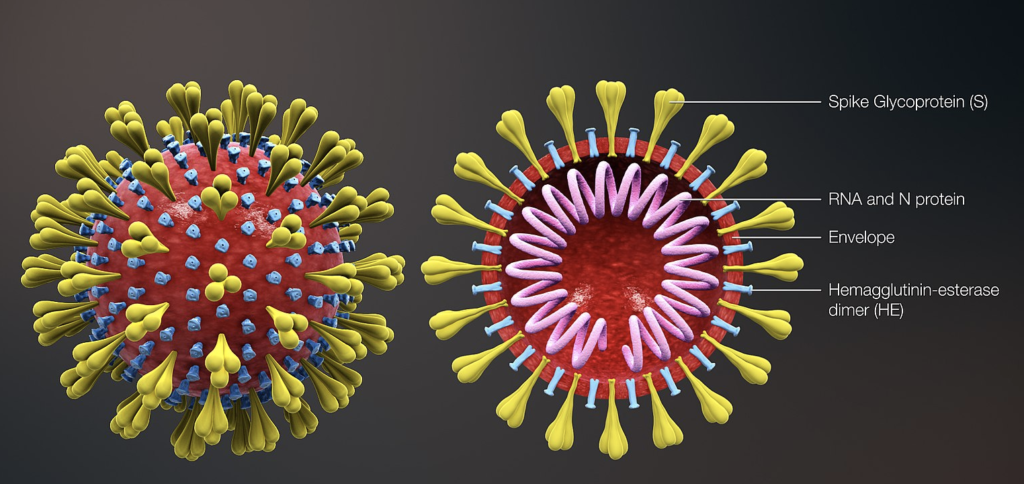
The coronavirus responsible for Covid-19 is SARS-CoV-2. It’s a so-called enveloped virus. It consists of a protein-bound single-stranded RNA molecule (= nucleocapsid) and an envelope formed by the membrane lipid structures of the cell in which it has multiplied. From this structure emerge “spikes”, protein structures appearing in the form of a crown (hence the name coronavirus) and which will serve as an inking point on the cellular receptors. It measures between 100 and 150 nm.
A virus is unable to reproduce itself. It enters a living cell to force it to make viruses in its own image. At the end of the process, the viruses escape from the depleted cell. They will then attack nearby cells to multiply. The disease is the consequence of the destruction of the cells, and therefore of the tissues, sometimes aggravated by an inappropriate response of the immune system, which releases harmful substances that in turn attack the tissues.
Reproduction
To replicate in the target cell the virus must
1- Entering the cell
2- Release its RNA which will serve as a matrix to synthesize identical RNA molecules that will constitute the new viruses.
3- Synthesize the 16 proteins that will be taken into the newly synthesized viral particles with RNA.
4- Get out of the cell
Steps and strategies to inhibit the virus
1- Entering the cell
a- Membrane fusion inlet
As in a “key-lock” system, the virus will attach itself to our cells through its spikes, which it will attach to the receptors in our cells called ACE2. Before attachment occurs, the spikes must be “activated” by a protease that splits them into two parts. This protease is itself present on the membrane of our cells and is called TMPRSS2.
Attachment is followed by fusion of the virus membrane and the cell membrane allowing RNA to enter the cell.
In summary: The spike is activated by the enzyme TMPRSS2 and can attach to the ACE2 receptor.
Antiviral strategies:
Blocking the spikes with antibodies found in the plasma of people cured of Covid-19: this is “plasma therapy”.
Blocking the activity of the enzyme responsible for activating the spike, TMPRSS2 by a drug called Camostat mesylate.

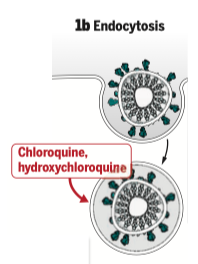
b- Entry by endocytosis
Like many enveloped viruses, the virus can also enter the cell by being totally absorbed into a vacuole: this is “endocytosis”. The vacuole is formed after contact with the membranes, again as a result of the activation of the spikes by TMPRSS2. The virus must then exit the vacuole in order to be able to “undress” itself inside the cell, i.e. get rid of its envelope and release the RNA from the proteins surrounding it. This step requires a variation in pH. This is where chloroquine (or the less toxic hydroxychloroquine) comes in to prevent this pH variation, thus preventing the entry of the virus. Its association with azithromycin, an antibiotic used against intracellular bacteria, would allow a potentiation of its effect.
2- RNA synthesis of future viruses
The viral RNA that has entered the cell is copied by an enzyme originally present in the virus: RNA polymerase.
A drug developed against the EBOLA virus and called “Remdesivir” is able to block the synthesis of these RNAs by inducing errors that cannot be repaired by the correction systems that exist in this virus.
3- Synthesis of proteins that will be carried away by the virus
Viruses consist of a genome that is RNA and 16 proteins that it carries with it (including the RNA polymerase seen above).
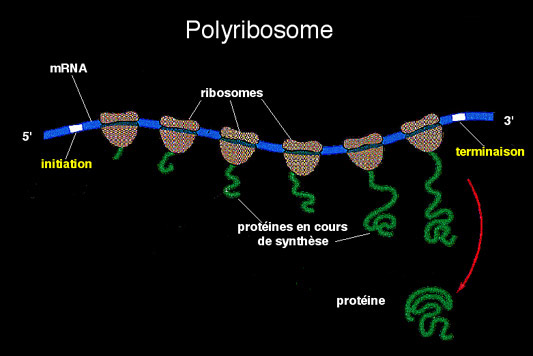
Not having the ability to synthesize proteins on his own, he will use the synthesis apparatus of the infected cell: the ribosomes.
The synthesis code is “messenger RNA” which has also been synthesized from viral RNA.
The cell’s ribosomes move over the messenger RNA and add the right amino acids end to end to form the protein.
So it is indeed the cellular machinery that synthesizes the proteins of the virus.
In reality, it is a large protein that is synthesized before being cut into pieces that will be the 16 proteins carried by the virus. This cutting is carried out by a viral protease of which an inhibitor has been developed to fight against the AIDS virus: it is the association “Lopinavir-ritonavir” (Kaletra), the lopinavir inhibits the protease while the ritonavir protects the lopinavir from a too fast degradation.

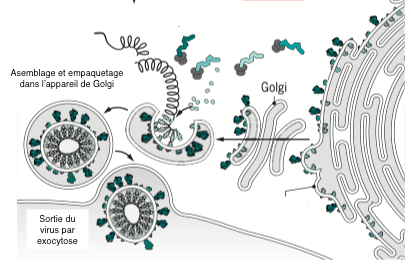
The different elements, RNA and proteins, assemble spontaneously in the cytoplasm of the cell and are then packaged in the golgi apparatus. The new viruses are rejected into the external environment, taking cell membrane with them. This is why they are called enveloped viruses. None of these steps are targeted for antiviral treatment.
The other strategies
Many other avenues are being explored such as :
To have monoclonal antibodies against spikes act instead of the natural antibodies in the plasma of cured patients;
Add an immune stimulant such as interferon beta to the Lopinavir- ritonavir mixture;
Boost the immune system by vaccinating the sick patient with BCG (Bacille Bilié de Calmette et Guérin) well known for its immunostimulant properties …
A clinical trial called Discovery started on March 23rd in France to test 4 experimental treatments. It is led by Florence Ader, an infectiologist in the Infectious and Tropical Diseases Department at the Croix-Rousse Hospital at Lyon University Hospital and a researcher at the CIRI International Research Centre for Infectious Diseases (Inserm/CNRS/University Claude Bernard Lyon 1).
The objective is to evaluate the efficacy and safety of four experimental therapeutic strategies that could have an effect against Covid-19 based on current scientific data.
The DISCOVERY trial starts with 5 treatment modalities:
standard care ;
standard care plus remdisivir;
standard care plus lopinavir and ritonavir;
standard care plus lopinavir, ritonavir and interferon beta;
standard care plus hydroxychloroquine.
This work includes 3,200 hospitalized patients throughout Europe, including 800 in France. This clinical trial is evolving. If one of the selected molecules is ineffective, it is abandoned. Conversely, if one of them works on one of the patients, it can be tested on all patients in the trial.
This trial will complement the SOLIDARITY study launched with more or less the same molecules since March 20 by the WHO on several thousand patients in different countries.
It will be noted that the molecules tested are known as well as their toxic effects, which will make it possible to go much faster in their implementation when the results are known.
Conclusion
Finding a treatment to prevent respiratory complications leading to death in Covid-19 patients is an absolute emergency. It was therefore decided to test molecules already on the market whose toxic side effects are well known. The results of these tests should not be long in coming.
Other teams are working on a vaccine that will protect people when the disease returns. Development is difficult and time-consuming, but essential to avoid this kind of pandemic, which has not occurred since the influenza pandemic of 1918-19.
Autor: Prof. François Renaud



